Developments in Understanding Diastaticus

Saccharomyces cerevisiae variety diastaticus, also know as S. cer. var. diastaticus or diastatic S. cerevisiae, has been a frequent topic of conversation within the brewing industry (Read our first report on the topic from 2018). This species of yeast is most notably used in Belgian style beers including the saison, blond and pale ale, dubbel, tripel, golden and dark strong ale, and bière de garde, among modern hybrid styles like the Belgian or White IPA. Recent studies have revealed new information regarding the ability of S. cer. var. diastaticus to ferment complex carbohydrates such as dextrins or starches. Due to potential issues associated with the presence of wild S. cer. var. diastaticus in the brewing environment or the cross-contamination of commercial diastatic yeast, advanced methods for detecting the organism have also been introduced. Applying these recent developments while researching S. cer. var. diastaticus strains in the Wyeast Culture Collection resulted in the findings discussed here.
STA1 Gene Expression and the Super Attenuator Phenotype
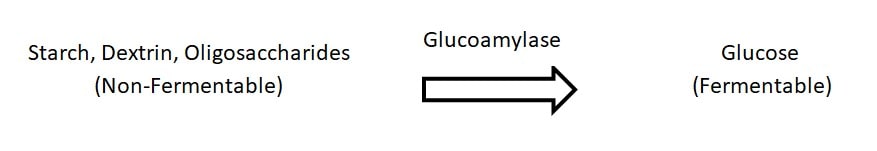
Figure 1. The conversion of non-fermentable polysaccharides to the fermentable monosaccharide, glucose, by glucoamylase.
The ability of S. cer. var. diastaticus to achieve high levels of attenuation is due to its ability to produce extracellular glucoamylase, which allows for fermentation of complex carbohydrates not metabolized by traditional strains of brewing yeasts, Saccharomyces cerevisiae or Saccharomyces pastorianus. This phenotypic trait causes S. cer. var. diastaticus strains to be referred to as “super attenuators”. The ability of S. cer. var. diastaticus to produce glucoamylase lies in its genome, specifically with the presence of STA genes. Detection of the STA1 gene through PCR analysis is the common method for classifying diastatic strains. Seven strains in Wyeast’s library are STA1 positive and have received the diastaticus classification as of 2017. These products are now identified as such on commercial and home enthusiast packaging, product information pages, certificates of analysis, and purchase invoices for complete, informative transparency.
Although all STA1 positive strains contain the gene for production of glucoamylase, researchers and brewers have observed that not all STA1 positive strains exhibit the same ability to ferment complex carbohydrates. Isolates of S. cer. var. diastaticus from yeast libraries and from breweries were previously investigated and about half of the isolates demonstrated the ability to fully ferment the dextrins and starches present in a fermented beer medium (Meier-Dornberg, 2018). Diastatic strains in the Wyeast library also show broad ranges of attenuation with some strains not demonstrating super-attenuator behavior when used for primary fermentations.
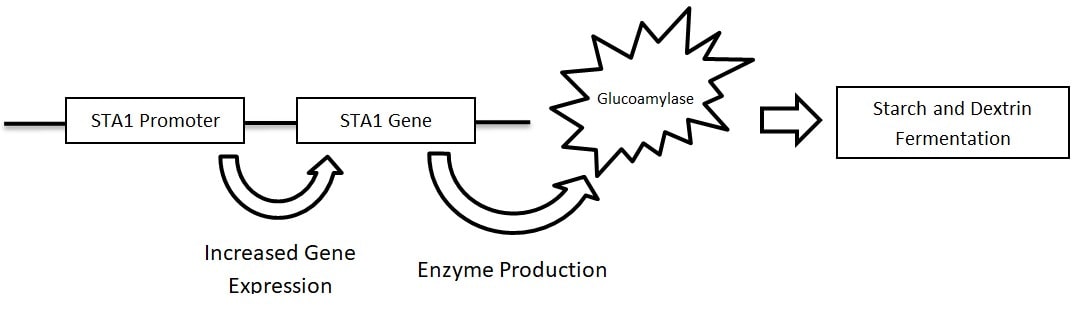
An explanation for the varied phenotypes of STA1 positive strains may be found by taking a closer look at their genome. The STA1 gene contains a promoter, which regulates expression of the gene and subsequent production of the enzyme, glucoamylase (Krogerus, 2020) (Figure 1). It was recently discovered that numerous strains of S. cer. var. diastaticus have deletions within the STA1 promoter sequence, causing decreased expression of the gene (Krogerus, 2019). Deletion of the promoter was also found to correlate with the strain’s ability to metabolize dextrins in beer and grow in mediums containing starch as a sole carbohydrate. In summary, S. cerevisiae var. diastaticus which have deletions in their STA1 promoter have reduced ability to ferment complex carbohydrates and cause issues associated with over-attenuation.
Figure 2. Activation of the STA1 gene by the STA1 promoter resulting in fermentation of complex carbohydrates.
PCR analysis of the seven strains of S. cer. var. diastaticus in the Wyeast library revealed that only three strains contain the STA1 promoter: 3711 French Saison, 3739-PC Flanders Golden Ale, and 3725-PC Bière de Garde. Understanding the role of the promoter, it is expected that these three strains will demonstrate super attenuator behavior in primary fermentations. When all STA1 positive strains were used to ferment 15 oP wort at 30 oC (86 oF) for fourteen days, these three strains showed the highest levels of apparent attenuation, with values between 86 and 90% (Table 1). In contrast, all strains without the promoter had lower levels of apparent attenuation, with values ranging between 75 and 85%.
|
Strain |
STA1 | STA1 Promoter |
Apparent Attenuation |
| 3711 | + | + |
90 |
| 3739 | + | + |
89 |
| 3725 | + | + |
86 |
| 3726 | + | – |
84 |
| 3822 | + | – |
81 |
| 3724 | + | – |
77 |
| 1388 | + | – |
75 |
| 1056 (control) | – | – |
78 |
Table 1. STA1 positive strains with functional STA1 promoters show the highest levels of apparent attenuation during primary fermentation of standard wort. 1056 American Ale was used as a control.
As knowledge regarding expression of the STA1 gene improves, so does our understanding of the fermentation behavior of S. cer. var. diastaticus. By identifying S. cer. var. diastaticus strains that contain the STA1 promoter we hope to provide a more comprehensive understanding of how these strains will perform during fermentation and in the brewery environment.
Monitoring S. cerevisiae var. diastaticus
When conducting fermentations with S. cer. var. diastaticus, it is important to implement monitoring procedures to validate sanitation practices and detect cross-contamination events. Employing a combination of both microbiological plating and molecular testing through PCR is suggested. Luckily, there are commercially available products which assist brewers in conducting these tests.
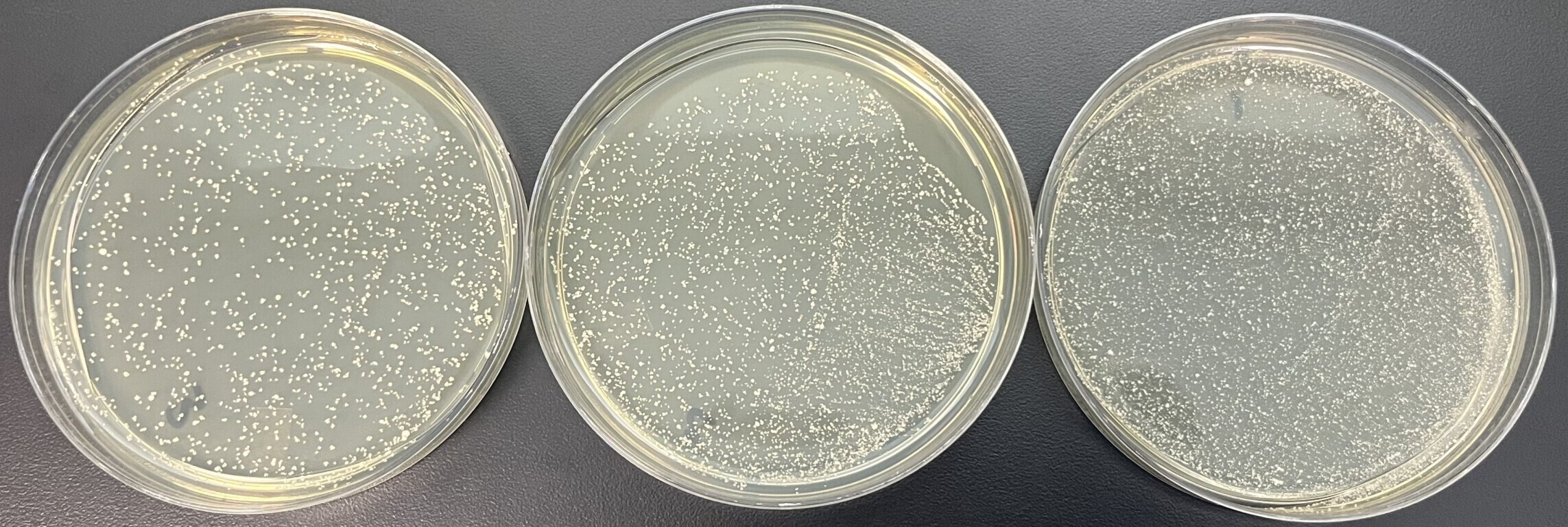
Differential media for detection of wild yeast in the brewery have been around for decades and still remain a viable method for detecting S. cer. var. diastaticus (Lin 1975). One differential media, Lin’s Cupric Sulphate Medium (LCSM) is commonly used for microbiological testing of samples in the brewery. LCSM was formulated to detect non-Saccharomyces wild yeast by inhibiting growth of Saccharomyces with cupric sulphate but also detects wild and commercial strains of S. cer. var. diastaticus. Although the presence of the STA1 gene is unrelated to a yeast’s resistance to copper ions, this media can be used for detecting S. cer. var. diastaticus (Figure 3). All S. cer. var. diastaticus strains in Wyeast’s culture collection are able to grow on commercially available formulations of LCSM (Table 2).
Microbiological plating is an accessible method for detecting viable cells of S. cerevisiae var. diastaticus but it does have shortcomings. These media require strict adherence to the manufacturer’s instructions and do not provide immediate results. Other species of wild yeast can also grow on LCSM and it is important to perform further analysis on colonies to identify the organism, especially when a brewery employs multiple strains or species for fermentation.
| Strain | STA1 |
LCSM |
|
3711 |
+ | + |
| 3739 | + |
+ |
|
3725 |
+ | + |
|
3726 |
+ |
+ |
| 3822 | + |
+ |
| 3724 | + |
+ |
| 1388 | + |
+ |
| 1056 (control) | – |
– |
Table 2. Growth of STA1 positive strains on the differential media, LCSM.
Figure 3. Growth of an STA1 positive yeast strain at varied concentrations on LCSM.
PCR detection of the STA1 gene is the accepted method for identifying S. cerevisiae var. diastaticus and it is also used for screening brewery samples for the presence of the organism. Conducting in-house PCR analysis has become more affordable and available to brewers in recent years and provides some benefits over traditional plating. This technology allows for rapid detection of S. cer. var. diastaticus in a few hours, so long as it is present in concentrations above the limit of detection. However, it is of critical importance to understand that PCR detects the presence of genetic material – which can come from a living viable cell or non-viable DNA remnants – and should not be interpreted as a live contaminant unless also confirmed by microbiological plating.
Every culture of yeast propagated by Wyeast Laboratories is analyzed through microbiological plating on wild yeast media and screened with our PCR system to assure products are free of contaminants as part of our finished product protocol and product specifications. As continued research is performed on S. cerevisiae var. diastaticus and new technologies emerge for monitoring the organism, we will continue to share information about our strains and improve our quality control protocols.
References
Krogerus, K.; Gibson, B. (2020) A re-evaluation of diastatic Saccharomyces cerevisiae strains and their role in brewing. Applied Microbiology and Biotechnology 104:3745–3756.
Krogerus, K.; Magalhaes, F.; Kuivanen, J.; Gibson, B. (2019) A deletion in the STA1 promoter determines maltotriose and starch utilization in STA1+ Saccharomyces cerevisiae strains. Applied Microbiology and Biotechnology 103:7597-7615.
Lin, Y. (1975) Detection of Wild Yeast in the Brewery – Efficiency of Differential Media. Journal of the Institute of Brewing 81(5) 410-417.
Meier-Dornberg, T.; Jacob, F.; Michel, M.; Hutzler, M. (2017) Incidence of Saccharomyces cerevisiae var. diastaticus in the Beverage Industry: Cases of Contamination, 2008–2017. MBAA TQ 54(4) 140-148.
Meier-Dornberg, T.; Kory O.I.; Jacob, F.; Michel, M.; Hutzler, M. (2018) Saccharomyces cerevisiae variety diastaticus friend or foe?—spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization. FEMS Yeast Research 18(4).